Arrowhead Medical Device Technologies pierces patent challenge
l device startup
By Kevin McKenzie
Originally published 06:32 p.m., March 26, 2014
Updated 06:49 p.m., March 26, 2014
For Arrowhead Medical Device Technologies LLC, winning a first patent is a milestone too often overlooked in the medical device manufacturing industry.
“Having a patent issued is a big milestone,” said Patrick Mullaney, Arrowhead’s president. “It’s a big milestone that all too often is unappreciated and not respected.”
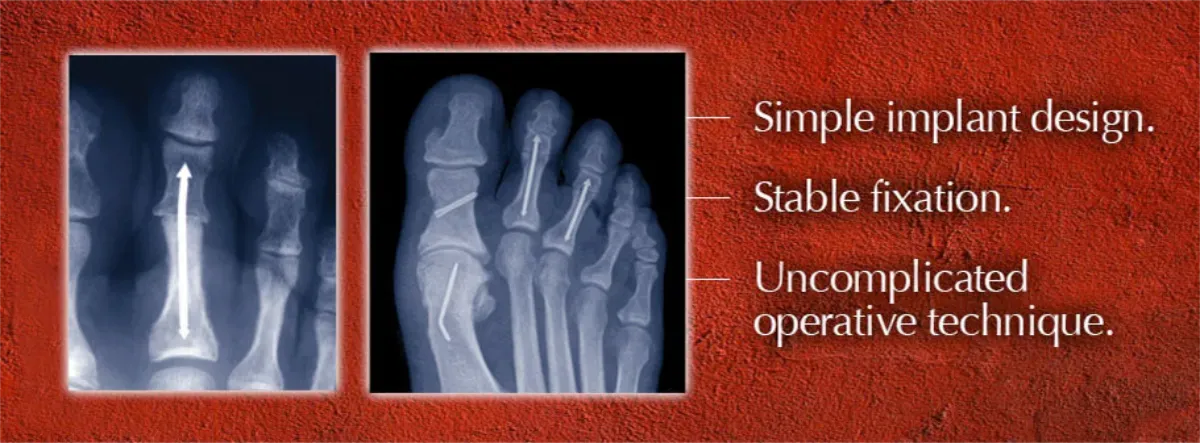
On Tuesday, a new patent for the company’s signature product — a thin, stainless steel rod with arrowhead-shaped tips at both ends that is implanted to fuse toe joints — will be issued, said Tom Twardzik, company co-founder and vice president of marketing and operations.
Invented by an Atlanta-area surgeon, Dr. Scott R. Roman, the patent for “intramedullary fixation device and methods for bone fixation and stabilization” has been four years in the making and provides legal protection from copy cats.
“It really does substantiate the company when you do get a patent,” Mullaney said. “There are companies that operate with no patents.”
Mullaney, 46, and Twardzik, 57, founded Arrowhead in 2010. Both are former employees of Smith & Nephew, the London-based medical device manufacturer with operations in Memphis.
Mullaney, in sales at Smith & Nephew beginning in 1991, left in 2004 to work for another orthopedic device maker. Twardzik worked in marketing and was “downsized,” rehired and downsized again.
With private investors in Memphis and in Georgia, the company has invested close to $3 million in the last three years making and marketing its Arrow-Lok digital fusion system for hammer toe, which joint condition that causes toes to be bent like a hammer.
The company’s products have been used in more than 2,000 procedures in a market where 500,000 are done a year, the executives said.
Arrowhead Medical Device Technologies pierces patent challenge : Memphis Commercial Appeal
Dr. Paul Hutchison, a Memphis podiatrist, said the company product is the primary fixation device he now uses for hammer toes.
“What you’re looking for is ease of usage, but reproducible results,” Hutchison said. “This product has entitled me to have that,” said Hutchison, who also described it as awesome, neat and innovative.
Mullaney, Twardzik and one sales-related position form the company’s full-time staff. About 80 independent field representatives, who also market products for other firms, spread the word about the company’s products to physicians in the region.
Mullaney, who helped found a previous medical device startup that was sold and learned business basics running a M r. Ding-A-Ling ice cream franchise with his brothers in New York, said starting up a medical device company is very difficult.
But he and Twardzik counted off former employees of Smith & Nephew and related firms in Memphis that provide a foundation for medical device startups.
“The investment made in creating a company, even a small company like ours, translates to millions of dollars into the economy here,” Twardzik said.
As printed in the Commercial Appeal